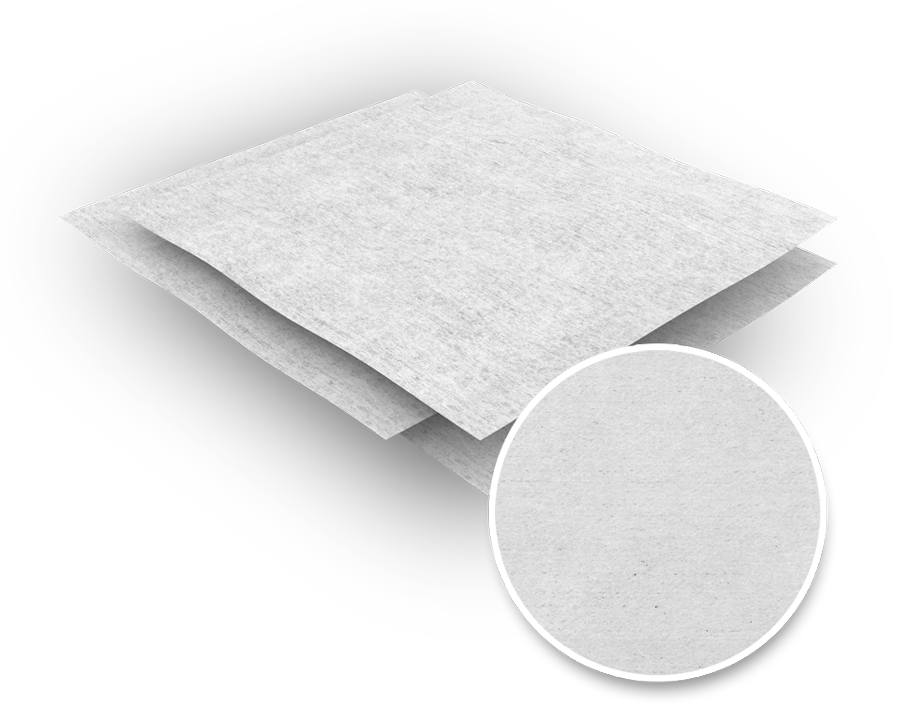
The Contec® Critical Site® Sterile Wipes are sterile, hydroentangled polyester non-woven wipes impregnated with 70% IPA / 30% DI water. Thanks to their small format, they are especially suitable for a range of wet cleaning tasks in areas where validated sterility and particulate cleanliness are required on the one hand, and for small and hard-to-reach surfaces, such as vials, on the other.
They are packaged in a user-friendly, resealable flat pouch designed for a greater need for wipes per use.
Facts
Getränkte Tücher
Non-woven, 100% PES, impregnated with 70% IPA / 30% DI water, validated sterile.
Properties
- hydroentangled non-woven fabric made of 100% polyester
- saturated with 70% IPA / 30% DI water
- validated sterile (SAL at 10-6)
- particulate clean
Advantages
- small wipe needed in larger quantities per use
- good chemical resistance
- reduced levels of volatile organic compounds (VOC)
- low-cost version compared to knitted wipes
- fast-drying disinfection
- storage costs for cleaning agents and time-consuming ancillary work such as decanting, spraying and wetting are eliminated
- resealable bag ensures constant solvent saturation during use
- space-saving, ideal for isolators or unidirectional airflow cabinets (UDAF)
Applications
- ideal for wiping the top of vials prior to piercing the septum for reconstitution or use
- pharmaceutical industry
- medical devices and equipment
- cleaning of parts of insulators or barrier systems with restricted access (RABS)
- removing contamination from the workplace and during maintenance work
Product recommendation based on cleanroom classes
Of course, cleanroom wipes used in ISO 5 can also be used in ISO 9, but in this case the economic efficiency and usefulness should be considered.
A 1 to 1 allocation of cleanroom wipes to an air cleanliness class according to ISO 14644-1 is not possible. Recommendations can only be made on the basis of special properties relevant from a cleanroom technical point of view, such as "abrasion resistance" or "particle emission". Users can find further information on this in VDI Guideline 2083 Part 9.2.
Technical data
| Properties | Unit of measurement | Value | Test method | |
|---|---|---|---|---|
| Material (fabric) |
100% PES hydroentangled | |||
| Saturation solution |
70% IPA / 30% DI water | |||
| Edge processing | cut | |||
| Mass per unit area | g/m2 | 67 | ||
| Thickness |
mils mm |
n.s. n.s. | ||
|
Non-volatile residues NVR |
IPA based DI water based |
ppm ppm |
0.008 0.055 |
IEST-RP-CC004.3, Sec. 7.1.2 IEST-RP-CC004.3, Sec. 7.1.2 |
| Particle residues | > 0.5 µm | x 106/m2 | 39.3 | IEST-RP-CC004.2, Sec. 5.1 |
| Fibre residues | > 100 µm | x 103/m2 | 10.4 | IEST-RP-CC-004.2, Sec. 5.2 |
| Ionic residues |
Sodium (Na+) Chloride (ClO2-) |
ppm ppm |
2.16 9.35 |
IEST-RP-CC004.3 IEST-RP-CC004.3 |
| Sterilisation | validated sterile | SAL (10-6) | yes |
AAMI ISO 11737-1 Sterilization of health care products - Radiation |
| Dimensions | PU per case | Art. no. | ||
|
4" x 4" (~ 10 x 10 cm) |
1,440 pieces (60 pcs./pouch) | 59802-01 |
Note