Width
Bacterial Filtration Efficiency
Particulate Filtration Efficiency
Pressure difference delta P
sterile
latex-free
three layer
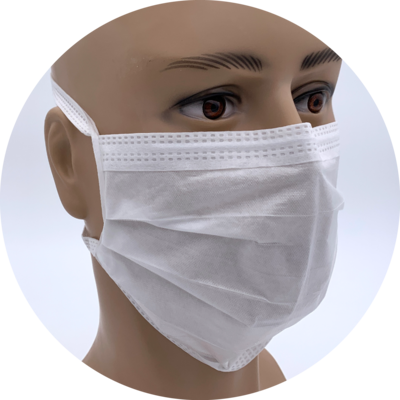
Disposable garments for the facial area
Kimtech™ M3, disposable face mask with soft ties, Art. No. 44830
23 cm
≥89% at 0.3 µm
≥84% at 0.1 µm
≤2.5 mm H2O/cm2
- sterile
- without natural latex
- under controlled conditions triple packed in PE bag
- aseptic filling and production
Product recommendation based on cleanroom classes
Air cleanliness class EN ISO 14644-1
particulate monitored areas
Hygiene zones according to GMP
microbiological monitored areas
A one-to-one classification of disposable face masks to a cleanroom class according to ISO 14644-1 is not possible. Recommendations can only be made based on cleanroom specific requirements, e.g. "abrasion resistance" or "particle emission" and, to some extent, „filtration efficiency“. In the VDI guideline 2083 Part 9.2 chapter 6 the user can find additional information.
Technical data
| Unit of measurement | Value | Test method | ||
| Type of material (face mask) |
spray coat/film* spunbonded fabric | |||
| Width (± 5 mm) | cm |
23 | ||
| Height (± 5 mm) | cm |
9.5 | ||
|
Materials (face mask) outer layer filter medium inner layer | polyethylene (PE) * polypropylene (PP) BICOSOFTM (PE) | |||
| Pleating |
three times | |||
| Type of fastening |
binding ties, flat hydro-entangled non-woven fabric | |||
| Materials (fastening) |
Polyester (PES) | |||
| Material nose bridge/iron |
completely wrapped, malleable aluminium strip | |||
| Seams |
thermally bonded | |||
| Particle filtration efficiency (PFE) | % at 0.1 µm | ≥84 |
ASTM F2299 Standard Test Method for Determining the Initial Efficiency of Materials Used in Medical Face Masks to Penetration by Particulates Using Latex Spheres | |
| Bacterial filtration efficiency (BFE) | % at 0.3 µm | ≥89 |
ASTM F2101 Standard Test Method for Evaluating the Bacterial Filtration Efficiency (BFE) of Medical Face Mask Materials, Using a Biological Aerosol of Staphylococcus aureus | |
| Breathability / differential pressure (∆P) | mm H2O/cm2 | ≤2.5 |
MIL-M-36954C U.S. Department of Defence test method for disposable surgical masks | |
| Viral filter efficiency (VFE) | % per x l/min A. | n.s. | ||
|
Airborne Particles (APC by Helmke Drum) | ≥0.5 µm | Cat. I (<460) |
IEST-RP-CC003.4 Garment System Considerations for Cleanrooms and Other Controlled Environments | |
| Sterility | SAL value | 10-6 | ANSI / AAMI / ISO 11137 | |
| Colour (face mask/band) |
white/white | |||
| PU |
200 pieces in a cardboard, 10 bags of 20 pieces, individually foil-packed |